Scientists at the Illinois Institute of Technology and Argonne National Laboratory have developed a new approach based on a four-electron reaction process to produce lithium-air batteries that have higher energy density than current Li-ion technology.
So far, lithium-air battery demonstrations have been limited to only one- or two-electron reaction processes, resulting in the formation of lithium superoxide (LiO2) or lithium peroxide (Li2O2), respectively. The reaction in the four-electron reaction process relies on the use of a solid-state electrolyte and a catalyst, trimolybdenum phosphide (Mo3P). This is important, as reactions involving more electrons create more energy storage.
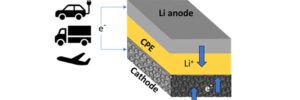
The scientists have created a lithium-air battery cell consisting of a lithium metal anode, air-based cathode and solid ceramic polymer electrolyte (CPE). Upon discharge and charge, lithium ions (Li+) go from anode to cathode, then back.
Unlike traditional lithium-ion batteries that use liquid electrolytes, the new battery uses a solid composite electrolyte based on nanoparticles containing lithium. The electrolyte is embedded in a matrix made of a special material called a ceramic-polyethylene oxide polymer. Researchers showed that the battery can be recharged for at least 1,000 charge-discharge cycles. With further development, the design could reach a specific energy of 1,200 Wh/kg.
The composite electrolyte embedded with Li10GeP2S12 nanoparticles exhibits high ionic conductivity and stability and high cycle stability. Low-dose cryogenic transmission electron microscopy carried out at the Center for Nanoscale Materials, a DOE Office of Science user facility, confirms the reaction mechanism, which favors four-electron reaction chemistry by reversible formation and decomposition of Li2O as the main product.
The research found that the battery is rechargeable for at least 1,000 cycles at room temperature, which the scientists say represents significant progress toward practical applications of lithium-air batteries.
Using a solid-state electrolyte instead of a liquid electrolyte would also reduce concerns around fire safety. The discovery also opens up novel ideas for designing lithium-based battery chemistry that works at room temperature. These future designs could achieve even greater energy storage, according to the researchers.


